UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, DC 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934
Date of report (Date of earliest event reported): October 16, 2019
Catabasis Pharmaceuticals, Inc.
(Exact Name of Registrant as Specified in Charter)
|
Delaware |
|
001-37467 |
|
26-3687168 |
|
(State or Other Jurisdiction |
|
(Commission |
|
(IRS Employer |
|
One Kendall Square |
|
02139 |
|
(Address of Principal Executive Offices) |
|
(Zip Code) |
Registrant’s telephone number, including area code: (617) 349-1971
(Former Name or Former Address, if Changed Since Last Report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions (see General Instruction A.2. below):
o Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425)
o Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12)
o Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b))
o Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c))
Securities registered pursuant to Section 12(b) of the Act:
|
Title of each class |
|
Trading Symbol(s) |
|
Name of each exchange on which |
|
Common Stock, $0.001 par value per share |
|
CATB |
|
The Nasdaq Stock Market LLC |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging growth company x
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. x
Item 7.01. Regulation FD Disclosure
On October 17, 2019, Catabasis Pharmaceuticals, Inc. (the “Company”) is making publicly available on its website an updated corporate slide presentation. The updated slide presentation is furnished as Exhibit 99.1 to this Current Report on Form 8-K and is incorporated herein by reference.
The information in this Current Report on Form 8-K, including Exhibit 99.1, shall not be deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934 (the “Exchange Act”) or otherwise subject to the liabilities of that section, nor shall it be deemed incorporated by reference in any filing under the Securities Act of 1933 or the Exchange Act, except as expressly set forth by specific reference in such a filing.
Item 9.01. Financial Statements and Exhibits.
(d) Exhibits
|
Exhibit |
|
Description |
|
99.1 |
|
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
|
|
CATABASIS PHARMACEUTICALS, INC. | |
|
|
|
|
|
Date: October 16, 2019 |
By: |
/s/ Jill C. Milne |
|
|
|
Jill C. Milne |
|
|
|
President and Chief Executive Officer |
October 2019 Our mission is to bring hope and life-changing therapies to patients and families affected by rare diseases Catabasis Pharmaceuticals

2 Forward Looking Statements This presentation contains, and any oral remarks made in connection with such presentation may contain, forward-looking statements within the meaning of The Private Securities Litigation Reform Act of 1995, including statements regarding our expectations and beliefs about our business, future financial and operating performance, clinical trial plans, product development plans and prospects, including statements about future clinical trial plans including, among other things, statements about our single global Phase 3 PolarisDMD trial in Duchenne muscular dystrophy, or DMD, to evaluate the efficacy and safety of edasalonexent for registration purposes, our plans to continue to evaluate data from the open-label extension of our MoveDMD® clinical trial and from our GalaxyDMD open-label extension trial of edasalonexent for the treatment of DMD, and our plans to combine edasalonexent treatment with other DMD treatments such as gene therapy and other dystrophin-targeted approaches. The words “believe”, “anticipate”, “plans,” “expect”, “could”, “should”, “will”, “would”, “may”, “intend” and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. The forward-looking statements contained in this presentation and in remarks made during this presentation and the following Q&A session are subject to important risks and uncertainties that may cause actual events or results to differ materially from our current expectations and beliefs, including: uncertainties inherent in the initiation and completion of preclinical studies and clinical trials and clinical development of our product candidates; availability and timing of results from preclinical studies and clinical trials; whether interim results from a clinical trial will be predictive of the final results of the trial or the results of future trials; expectations for regulatory approvals to conduct trials or to market products, including our expected target product profile for edasalonexent in DMD; availability of funding sufficient for our foreseeable and unforeseeable operating expenses and capital expenditure requirements; other matters that could affect the availability or commercial potential of our product candidates; and general economic and market conditions and other factors discussed in the “Risk Factors” section of our Quarterly Report on Form 10-Q for the period ended June 30, 2019, which is on file with the Securities and Exchange Commission, and in other filings that we may make with the Securities and Exchange Commission in the future. In addition, the forward-looking statements included in this presentation represent our views as of the date of this presentation. We anticipate that subsequent events and developments will cause our views to change. However, while we may elect to update these forward-looking statements at some point in the future, we specifically disclaim any obligation to do so. These forward-looking statements should not be relied upon as representing our views as of any date subsequent to the date of this presentation.

Catabasis and Edasalonexent: A Compelling Opportunity in DMD 3 Potential New Foundational Therapy in Duchenne Muscular Dystrophy (DMD) Promising disease-modifying oral NF-B inhibitor Slowed disease progression compared to off-treatment control period in MoveDMD trial Fast Track, Rare Pediatric, and Orphan Drug designations from FDA Orphan Medicinal Product designation from European Commission Pivotal Phase 3 PolarisDMD trial fully enrolled, top-line results expected in Q4 2020 NDA filing expected in 2021 Significant Commercial Opportunity Potential differentiated foundational treatment for all DMD patients High unmet medical need in clear target market with strong patient advocacy and concentrated Centers of Excellence Unique mechanism could enable use as mono- or potentially as combination therapy with other treatments such as exon skipping, gene therapies and other approaches Market research indicates high likelihood of physician adoption and payer coverage Expansion in DMD and Beyond Additional trial planned in non-ambulatory DMD patients Leverage benefits of inhibiting NF-B in other potential indications Leadership Depth and Focus Accomplished industry, financial and clinical leaders Seasoned team with experience in rare diseases and commercialization Strong IP position and wholly-owned assets

4 Edasalonexent: Potential for Broad Therapeutic Benefit Loss of ambulation, upper limb function, respiratory failure Skeletal Muscle Heart Cardiomyopathy Fractures Bone In DMD, the loss of dystrophin leads to chronic activation of NF-B, which is a key driver of skeletal muscle and cardiac disease progression Activated NF-B leads to disease progression in DMD Potential for edasalonexent, an NF-B inhibitor Goal: Improve skeletal muscle function Goal: Preserve cardiac function Goal: Reduce risk of fractures

5 Edasalonexent: Potential to Slow Disease Progression for All Those Affected by DMD Our Vision for Edasalonexent Foundational therapy for all DMD patients, regardless of mutation, from time of diagnosis onwards Address skeletal and cardiac muscle disease and bone health As monotherapy and potential to be used with: Other therapies, including exon-skipping and gene therapies Favorably differentiated safety and tolerability profile from other treatments Commercial Approach Disease-focused specialty sales force in US Establish global “go-to-market” strategies Developing a potential foundational therapy in DMD Edasalonexent is an investigational agent not currently approved in any territory
6 Fully Enrolled Edasalonexent Phase 3 PolarisDMD Trial Designed for Global Registration Goal: Validate results from MoveDMD trial Eligibility: All mutations Age 4 to 7 (up to 8th birthday); off steroids for >6 months Boys on a stable dose of eteplirsen were eligible to enroll Endpoints: Consistent with regulatory guidance Primary: Change in North Star Ambulatory Assessment Key secondary: Age-appropriate timed function tests Additional assessments include growth, cardiac and bone measures Primary Endpoint 12-month, randomized, double-blind placebo-controlled trial (n=131) Open-label extension Edasalonexent Placebo Edasalonexent 100 mg/kg

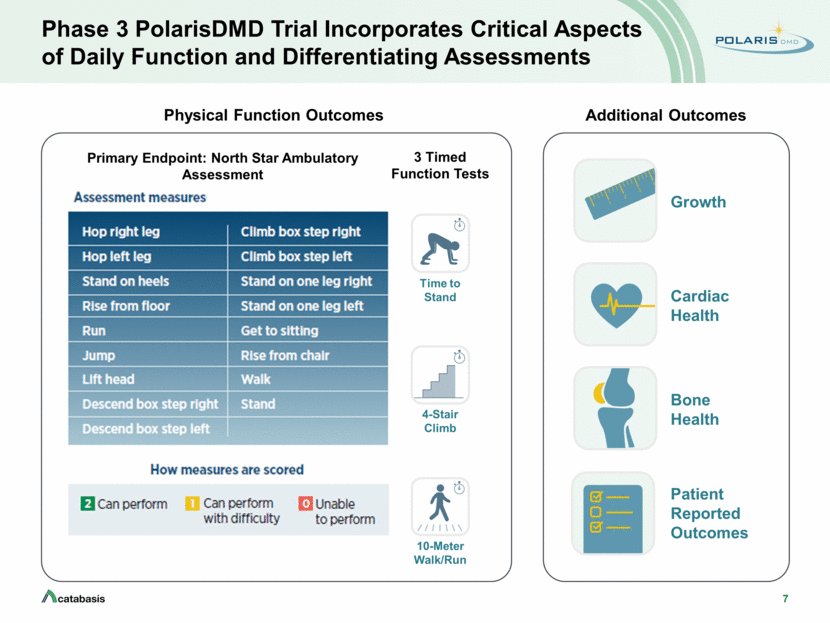
7 Phase 3 PolarisDMD Trial Incorporates Critical Aspects of Daily Function and Differentiating Assessments Physical Function Outcomes Primary Endpoint: North Star Ambulatory Assessment Additional Outcomes Time to Stand 4-Stair Climb 10-Meter Walk/Run 3 Timed Function Tests Cardiac Health Bone Health Growth Patient Reported Outcomes
8 PolarisDMD Was Designed Based on Promising MoveDMD Trial Results NF-B Target Engagement Biomarkers Muscle MRI Functional Inhibited NF-B targeted gene set in peripheral blood Decreased CK and other muscle enzymes Decreased CRP, biomarker of inflammation Improved rate of change in MRI T2 and MRS muscle fat compared to off-treatment control Preserved NSAA and Timed Function Tests In Phase 2 MoveDMD Trial and Open-Label Extension:

9 Edasalonexent Demonstrated Clinically Meaningful Slowing of Disease Progression North Star Ambulatory Assessment NSAA Score 25 20 15 10 5 -36 -24 -12 0 12 24 36 48 60 72 Average Rate of Change Edasalonexent 100 mg/kg Control Period Speed (1/Seconds) 0.4 Edasalonexent 0.3 0.2 0 -36 -24 -12 0 12 24 36 48 60 Average Rate of Change 100 mg/kg Control Period 0.1 4-Stair Climb 72 Time (Seconds) 5 10 15 10-Meter Walk/Run Speed (1/Seconds) Time (Seconds) 0.20 0.18 0.16 0.14 0.12 5 10 -36 -24 -12 0 12 24 36 48 60 72 Average Rate of Change Edasalonexent 100 mg/kg Control Period Better Weeks Weeks Weeks Time to Stand Speed (1/Seconds) Time (Seconds) 0.3 -36 -24 -12 0 12 24 36 48 60 72 Edasalonexent 100 mg/kg 0 0.2 0.1 5 10 Average Rate of Change Control Period 15 Means ± SEM shown. Includes data of all boys initially started on 100 mg/kg dose (n=16) with 11 boys participating through 72 weeks. Results are compared to the off-treatment control period changes measured prior to boys in the MoveDMD trial receiving 100 mg/kg edasalonexent. Weeks In Phase 2 MoveDMD Trial and Open-Label Extension:

10 Edasalonexent Demonstrated Benefits on Additional Measures In Phase 2 MoveDMD Trial and Open-Label Extension: Significantly improved rate of change of MRI T2 compared to off-treatment control period MRI T2 increases over time in DMD as inflammation and fat content of muscle increases and inversely correlates with functional abilities Benefits on development measures including growth and cardiac Height and weight increases were similar to unaffected boys Mean resting heart rate significantly decreased, approaching age-normative heart rate of ~92 beats per minute Cardiomyopathy is the leading cause of death in DMD Elevated resting heart rate is the initial manifestation of cardiac disease in DMD These differentiating measures are being further explored in Phase 3 Willcocks, et al, 2016, Ann Neurol. Means + SEM; mixed model comparison with off-treatment period * Week 12: p=0.002, n=16; Week 24: p=0.004, n=14; Week 36: p=0.032, n=13; Week 48: p=0.018, n=12; Week 72: p=0.052, n=9

11 Edasalonexent Was Well-Tolerated with No Safety Signals 55+ patient years of exposure Well-tolerated, with majority of adverse events mild in nature Most common related adverse event was diarrhea, generally mild and transient and did not require discontinuation No serious adverse events on treatment (one on placebo) No adverse trends in chemistry, hematology or measures of adrenal function (cortisol and ACTH) Muscle enzymes significantly decreased on edasalonexent, including CK, supporting a positive impact on muscle health In Phase 2 MoveDMD Trial and Open-Label Extension: Means ± SEM shown; * p<0.05 for change from baseline after 12 weeks Weeks on 100 mg/kg Edasalonexent IU/mL 0 -5000 -10000 -15000 -20000 -25000 Creatine Kinase * 12 36 72 48 24 60

12 DMD Patient Segmentation and Typical Progression Is Well Established and Understood Source: http://www.dmd-guide.org/en-US/, CureDuchenne; All ages represented in the progression timeline are generalized and approximations Diagnosed by Age 5 Affected boys show clinical signs and symptoms Early Ambulatory Gower’s Maneuver Waddling gait Maybe toe-walking Climbs stairs slowly Late Ambulatory Labored gait Losing ability to climb stairs and rise from floor Early Non- Ambulatory May be able to self-propel for some time Able to maintain posture May develop scoliosis Late Non- Ambulatory Upper limb function and postural maintenance is increasingly limited Declining respiratory function Cardiac disease manifested 2 - 5 years 4 - 7 years 8 - 12 years 12 + years

DMD Patient Prevalence Population Is Well-Defined Source: McDonald C., et al., Lancet Neurol. 2018, 17:389-91. * Some females do present with DMD, exact prevalence unknown Because Duchenne gene is found on the X-chromosome, it primarily affects males, while females are typically carriers Affects 1 in 3,500-5,000 Males* Worldwide Approximately 15,000 Males* in the US Approximately 19,000 Males* in the EU 13

14 Clear Market Need in DMD with Limited Treatment Options Currently, there is no cure for DMD Today, the majority of patients are treated with corticosteroids Despite broad market utilization, steroids have long-term negative consequences Only a small portion of the population can be treated with eteplirsen (US) or ataluren (EU) Current Landscape of medical management Steroids Deflazacort and Prednisone Known Benefits: Delayed loss of muscle function Known Side Effects: Osteoporosis with fractures Metabolic effects Weight gain, obesity Growth retardation Delayed puberty Cataracts Muscle atrophy Behavioral issues Cushingoid appearance Mutation Targeted Eteplirsen (US) and Ataluren (EU) Safe and tolerable Limited labels; no outcomes data Limited suitable patient populations (13% for each targeted population)

15 Research Shows Support for Edasalonexent for DMD Among Key Stakeholders Sources: Catabasis Market Research conducted by Health Advances, 2018 and Blueprint Partnership, 2019 Potential to meet the needs of the DMD community: Strong efficacy profile in MoveDMD Well tolerated and no safety concerns to date A potential treatment for all boys Use as mono- or potential combo-therapy Age appropriate development similar to peers Edasalonexent DMD Patient Advocates and Parent/Caregivers US Payer/ Reimbursement Research US DMD Physicians/ KOL Research

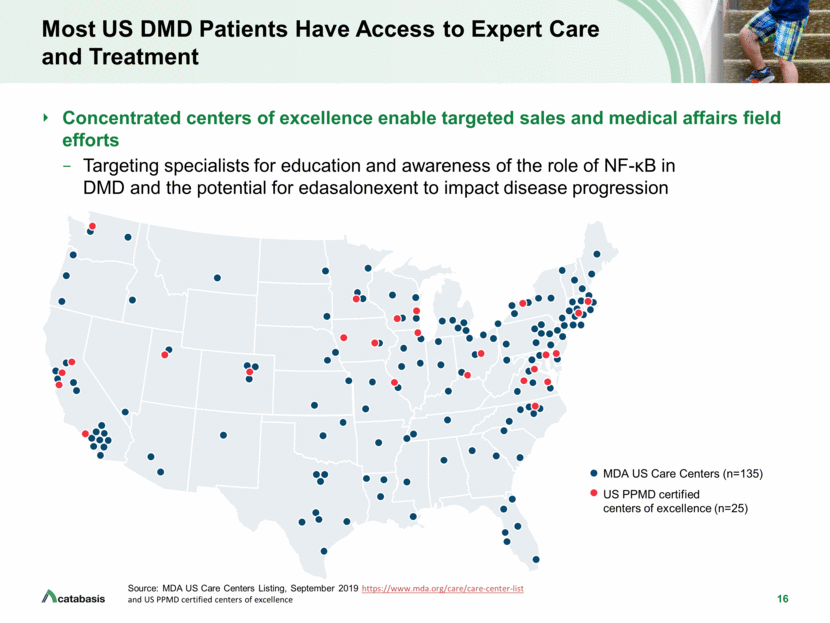
16 Most US DMD Patients Have Access to Expert Care and Treatment Concentrated centers of excellence enable targeted sales and medical affairs field efforts Targeting specialists for education and awareness of the role of NF-B in DMD and the potential for edasalonexent to impact disease progression Source: MDA US Care Centers Listing, September 2019 https://www.mda.org/care/care-center-list and US PPMD certified centers of excellence MDA US Care Centers (n=135) US PPMD certified centers of excellence (n=25)
17 Catabasis Has Developed Strong Relationships with Global DMD Patient Advocacy Organizations US Canada Sweden UK Israel Australia Europe Commitment to patients, caregivers and the community to help individuals with DMD Proud to partner with DMD Advocacy organizations to enroll Polaris Phase 3 Ongoing collaborations to drive education and awareness among treaters and payors

18 Actively Collaborating with Industry and Duchenne UK to Understand Burden of DMD Source: https://www.duchenneuk.org/project-hercules

19 Edasalonexent: Beyond Ambulatory DMD Potential Opportunities Demonstrate ability to be used in combination with dystrophin-targeted and next-generation therapies Expand clinical experience to all ages within the Duchenne community, including non-ambulatory patients Leverage benefits of inhibiting NF-B in other potential indications Combo with exon-skipping, gene therapy Other muscular dystrophies 60% of DMD patients are > 12 years 40% <12 Years DMD

20 Catabasis Is Striving to Improve the Lives of Patients Affected by DMD NF-B Targeted MOA Chronic activation of NF-B is a well-recognized driver of disease progression in DMD Edasalonexent inhibits NF-B and has a novel mechanism among the therapies available or in development for DMD with broad potential benefits Edasalonexent slowed disease progression with a favorable safety profile in MoveDMD trial Potential Foundational Therapy Potential for edasalonexent to be used as monotherapy or in combination with current and next-generation DMD treatments Oral therapy Favorable Market Profile Strong interest from physicians and KOLs Market research indicates high likelihood of physician adoption and payer coverage Potential to meet the needs and desires of the DMD community Relationship Focus Developing best-in-class internal capabilities and forming critical partnerships to execute a flawless clinical trial and subsequent launch Market Preparation Hired Chief Commercial Officer Commercialization planning underway

